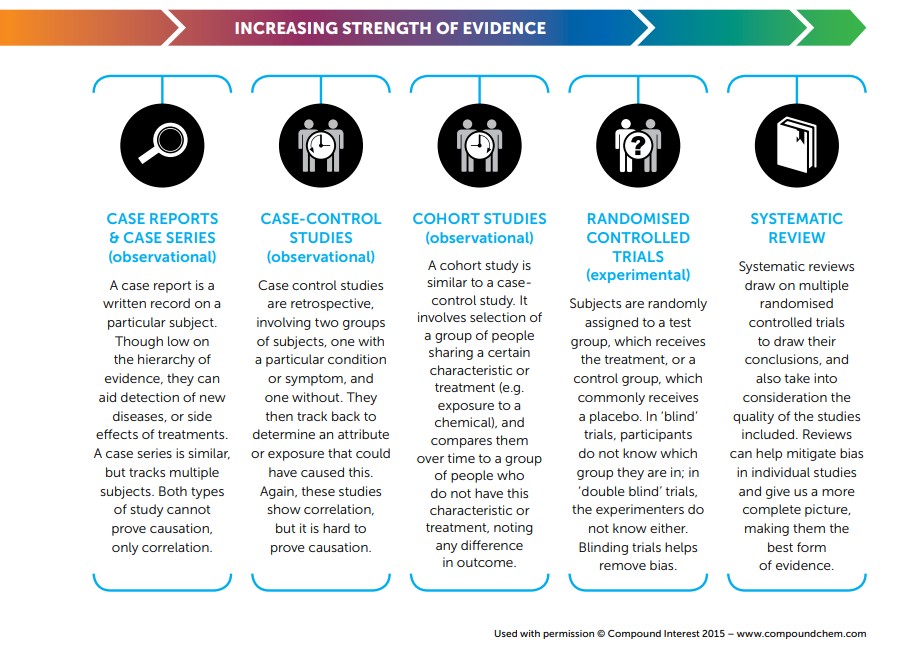
Being able to evaluate the evidence behind a claim is important, but scientific evidence comes in a variety of forms. Here, the different types of scientific evidence are ranked and described, particularly those relevant to health and medicinal claims.
Most scientific studies can be broken down into observational (observing something that happens) and experimental (which involve scientists controlling some of the variables). Generally, experimental studies are considered to provide stronger evidence and clearer cause and effect. This content is also available in our downloadable Desk Guide for Covering Science.
The types of evidence are listed from weakest to strongest.
Anecdotal & Expert Opinions
Anecdotal evidence is a person’s own personal experience or view, not necessarily representative of typical experiences. An expert’s standalone opinion, or that given in a written news article, are both considered weak forms of evidence without scientific studies to back them up.
Animal & Cell Studies (experimental)
Animal research can be useful, and can predict effects also seen in humans. However, observed effects can also differ, so subsequent human trials are required before a particular effect can be said to be seen in humans. Tests on isolated cells can also produce different results to those in the body.
Case Reports & Case Series (observational)
A case report is a written record on a particular subject. Though low on the hierarchy of evidence, they can aid detection of new diseases, or side effects of treatments. A case series is similar, but tracks multiple subjects. Both types of study cannot prove causation, only correlation.
Case-Control Studies (observational)
Case-control studies are retrospective, involving two groups of subjects, one with a particular condition or symptom, and one without. They then track back to determine an attribute or exposure that could have caused this. Again, these studies show correlation, but it is hard to prove causation.
Cohort Studies (observational)
A cohort study is similar to a case-control study. It involves selection of a group of people sharing a certain characteristic or treatment (e.g. exposure to a chemical), and compares them over time to a group of people who do not have this characteristic or treatment, noting any difference in outcome.
Randomised Controlled Trials (experimental)
Subjects are randomly assigned to a test group, which receives the treatment, or a control group, which commonly receives a placebo. In ‘blind’ trials, participants do not know which group they are in; in ‘double blind’ trials, the experimenters do not know either. Blinding trials helps remove bias.
Systematic Review
Systematic reviews draw on multiple randomised controlled trials to draw their conclusions, and also take into consideration the quality of the studies included. Reviews can help mitigate bias in individual studies and give us a more complete picture, making them the best form of evidence.
Note that in certain cases, some of these types of evidence may not be possible to procure, for ethical or other reasons.
Source for content: COMPOUND INTEREST 2015©, compoundchem.com. Used with permission.