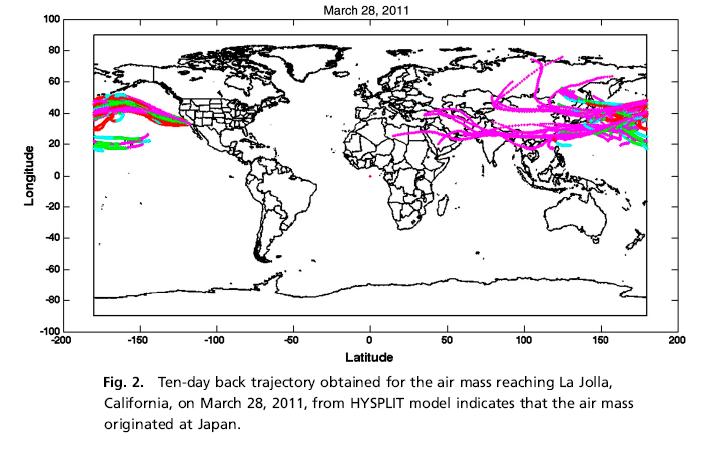
The journal Proceedings of the National Academy of Sciences (PNAS) has published a paper on radioactive sulphur detected in California, from the Fukushima nuclear plant.
 Scientists undertaking measurements in La Jolla, California, found that neutrons that leaked from the Fukushima nuclear reactor generated the radioactive sulfur, which was likely carried by strong westerly winds to southern California.
Scientists undertaking measurements in La Jolla, California, found that neutrons that leaked from the Fukushima nuclear reactor generated the radioactive sulfur, which was likely carried by strong westerly winds to southern California.
The authors estimate that close to 1% of the radioactive sulfate ions at the Pacific Ocean’s marine boundary layer, where sulfur dioxide is rapidly oxidized into sulfate ions, was carried in a plume to California.
The PNAS paper co-authors comment:
“Although the observed levels of radioactive sulfur are unlikely to harm human health, the findings demonstrate the usefulness of sulfur isotopes as markers for the transformation and long-distance transport of airborne chemicals.
Below are some comments on the research from scientists, gathered by our colleagues at the Science Media Centres in the UK and Canada. Please feel free to use these quotes.
From the UK Science Media Centre:
Professor Francis Livens, Professor of Radiochemistry, University of Manchester, comments:
“The term ‘neutron leakage’ used by the authors of this study may be misinterpreted by the general reader. The sulphur-35 that was detected is likely to have been generated in seawater (used as emergency cooling water in the reactors) by neutrons from spontaneous nuclear fission and nuclear absorption of alpha-particles – continuing nuclear fission chain reactions (the normal source of energy in a reactor) are not necessary to produce this level of neutrons. The paper represents a useful tracer experiment, but the sulphur-35 levels are low and of no radiological significance.”
Prof Jim Smith, Professor in Environmental Physics, University of Portsmouth, comments:
“The amounts of S-35 detected in the U.S. are tiny and are of no significance to human health. Even at Fukushima, the paper predicts only about 18 mBq of S-35 per cubic metre of air – many thousands of times lower than air concentrations of the much more important radiocaesium and radioiodine typically measured in the Fukushima region after the accident.”
Professor Paddy Regan, Professor of Nuclear Physics, University of Surrey, comments:
“This is an interesting paper which basically shows that neutrons produced in the core of the Fukushima reactors following their emergency shutdown (likely mostly beta-delayed neutrons from the fission fragments and some of the natural fission ones) interacted with specific material in the seawater (a naturally occurring isotope – Chlorine-35) which was used to cool the reactor down in the absence the normal water cooling (since the pumps were knocked out). This created the radioactive form of sulphur, (sulphur-35 or 35S). It needs to be noted that this radioisotope is also continuously created naturally from cosmic ray bombardments on the atmosphere. It is a useful ‘tracer’ nucleus because of its relatively short half-life (just under three months) which is short enough to be able identify / deduce where any increase source of this material have come from.
“The fact that the increased amount of 35S was seen for a few days in California following the airborne releases from Fukushima can be used to show the wind currents and speeds across the pacific ocean. This is very interesting from a scientific point of view, but people should certainly NOT read into it any radiation health scare in California from Fukushima. The levels were certainly increased and the sensitivity of the equipment showed that increase very clearly. However, the overall levels of this type of radiation compared to natural background are tiny and they pose no health risk. Also, the date shows (as expected), a spike in the increased 35S level followed by the expected decrease to approximate background levels over a few days.”
Professor Steve Jones, independent nuclear and environmental consultant, comments:
“Seawater used to cool the cores will have been exposed to a flux of neutrons sufficient to produce S-35, which will have been released to the environment in the same way as other radionuclides that have contaminated the seawater. Neutrons have not ‘leaked from the core’ – they would be present in any event along with the gamma, beta and alpha radiation from the fuel elements (which is of course why the core is heavily shielded). Quite possibly, other radioactive isotopes may have been produced by neutron activation of stable elements in the seawater, but most will have short half-lives and will not have persisted for long enough to be detected in the environment.”
Comments gathered by the Science Media Centre of Canada:
Anthony Waker, NSERC/UNENE Industrial Research Chair in Health Physics and Environmental Safety, University of Ontario Institute of Technology comments:
Is the paper significant?
“I think as far as atmospheric modelling goes, it’s quite significant. It’s an experiment they were suddenly able to do, so they did. The Oceanographic Institute was measuring sulphur anyway, because SO4 aerosol is a useful trace to study how the atmosphere and ocean work together. Suddenly, there was this event at Fukushima that released a lot of it, and it’s not often that you get a point event, something they could use to check their models. So they measured this stuff and used models to trace back to what they think was released at Fukushima.
One thing to note is they’re not coming from a radiological perspective. They’re really saying something about how SO4 interacts with the boundary layers of the atmosphere, which is significant in itself, but they don’t make any attempt at a radiological assessment.
What – if any – biological effects do these numbers suggest?
“I’ve done a few calculations here, and radiologically, they derived a number of sulphur atoms per metre cubed of air, and then back-estimated. From that, knowing the half-life of S35, I can calculate the activity of S35 per metre cubed [in California] and I derive a value of 0.00126 becquerels per cubic metre, where a becquerel is one instance of radioactive decay. The Canadian Nuclear Safety Commission has a radiation safety data sheet on S-35, on the annual limit of intake. The amount you could take into your body to arrive at a dose limit of 20 millisieverts. this translates to 200 million becquerels of S35.
“However, the limit for a member of the public is only 1 millisievert per year.
“So if you take that number and divide by 20, you come to 10 million becquerels, that you could take in to arrive at that 1 millisievert.
“If you take the concentration in California: [during light work, a person breathes] about 0.02 metres cubed [of air] per minute. Multiply that number by 0.00126 and my sums come out to 2.52 x10*-5 becquerels per minute.
“If that’s the rate of intake, per minute, then we would need to breathe that for about 3.9 x 10*11 minutes, and that is roughly 700,000 years.”