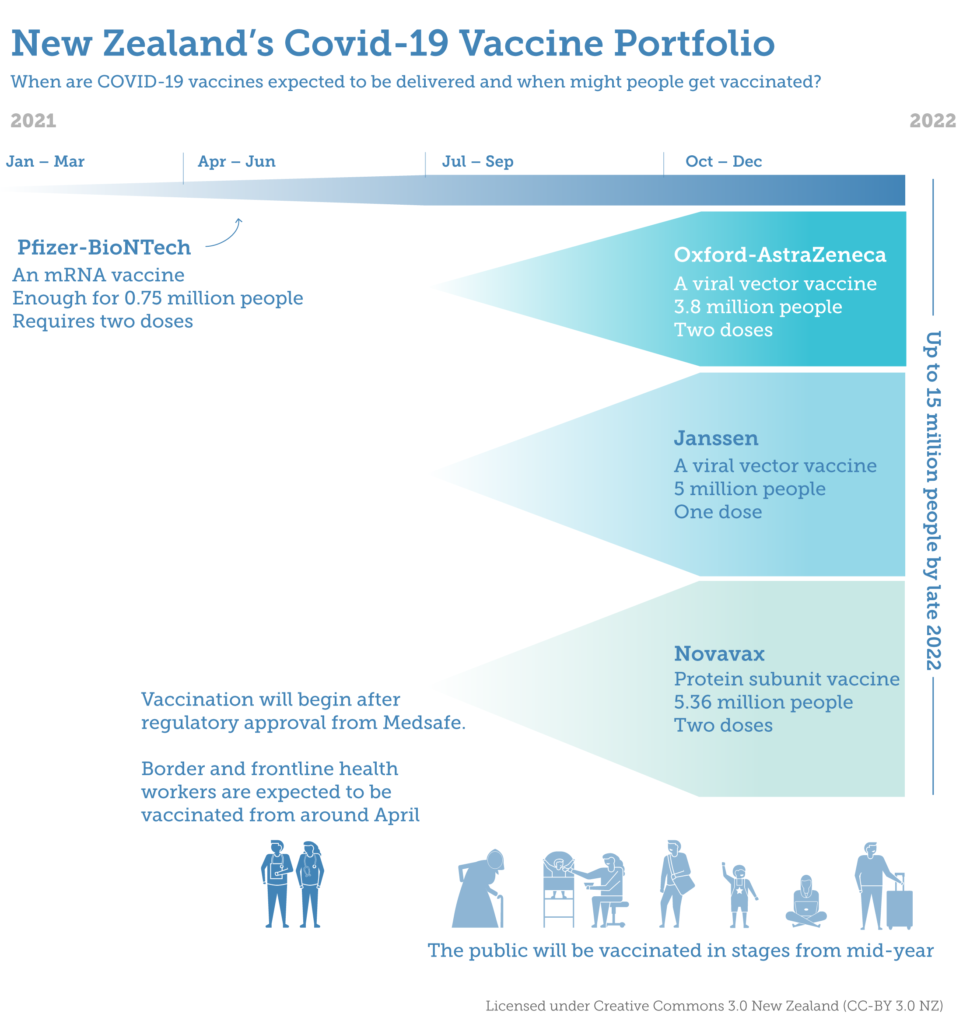
Border workers are expected to be the first group vaccinated for COVID-19 by winter, as enough jabs are pre-purchased to vaccinate every New Zealander for free.
The government has agreed to purchase 7.6 million doses from AstraZeneca (enough to vaccinate 3.8 million people) and 10.72 million doses from Novavax (for 5.36 million people). Today’s announcement builds on the government’s existing agreement to purchase 1.5 million doses of the Pfizer/BioNTech vaccine (for 750,000 people) and an in-principle agreement to purchase up to 5 million COVID-19 vaccines from Janssen Pharmaceutica (for 5 million people).
This graphic shows the timeline for each COVID-19 vaccine in the portfolio so far, as well as when people are expected to be vaccinated. (Click through for a full-res version)
The SMC also asked experts to comment on the announcement.
Professor Michael Baker, Professor of Public Health, University of Otago, Wellington, comments:
“It is very good to see these details about the next major step in New Zealand’s pandemic response. The speed and effectiveness of global vaccine development has been astounding, particularly the new class of RNA vaccines.
“These vaccines will make it much easier for New Zealand to sustain its elimination status as it transitions from use of non-pharmaceutical interventions (notably border quarantine) to use of vaccines instead.
“It is also good to see New Zealand’s continuing commitment to supporting Pacific and global vaccine access for low and middle income countries.
“There are still many unknowns, notably the duration of protection provided by these vaccines and their ability to interrupt transmission of the Covid-19 virus. But these uncertainties should be manageable in various ways.
“It is important that this announcement doesn’t add to complacency about the pandemic threat. New Zealand is now entering a high risk period over summer when we will continue to see large numbers of imported cases arriving into our MIQ facilities from overseas countries where the pandemic is still increasing.
“I would like to see New Zealand put much more effort into managing this risk by introducing measure to reduce the number of such infected travellers arriving here (eg using short periods of quarantine and testing in those source countries, which could be tracked using our booking system).”
No conflict of interest.
Dr Fran Priddy, Clinical Director Vaccine Alliance Aotearoa New Zealand – Ohu Kaupare Huaketo, comments:
“This is a savvy strategy for several reasons. The government has selected, in general, large, experienced pharmaceutical companies that know how manufacture, gain regulatory approval and distribute high quality products at scale. They have followed the science and are including multiple vaccine technologies. There are multiple back ups built into the plan, since there are 4 vaccines and enough doses for at least 2-3 of the vaccines to cover all NZers. The Covax investment provides an additional back-up strategy is more vaccine doses or the unlikely event that a fifth or different vaccine is needed.
“The strategy is also thinking about the region, including vaccines with cold chain that will be appropriate for Pacific neighbors. Pfizer and AstraZeneca vaccines have already demonstrated efficacy, and it is reasonable to assume that the Janssen adenoviral vector will be similarly effective (since the AstraZeneca vaccine is also adenoviral vector technology) and that protein vaccines like Novavax using the spike protein antigen will also be effective.
“The roll-out strategy is logical in considering not just our current scenario but potentially worse scenarios over the next year plus. Given New Zealand’s enviable position of having minimal cases and no community spread currently, focusing on border personnel and health care workers is a very reasonable strategy for initial vaccine use.”
Conflict of interest statement: Dr Priddy is Clinical Director of the Government-funded Vaccine Alliance Aotearoa New Zealand – Ohu Kaupare Huaketo, a partnership between the Malaghan Institute, the University of Otago and Victoria University of Wellington.
Dr Nikki Turner, Director Immunisation Advisory Centre, University of Auckland, comments:
“New Zealand is taking a sensible approach to pre-purchase vaccines that are looking promising with good science behind them. To date the early data on the leading-edge vaccine contenders are looking extremely promising, but we are still waiting for further scientific data before we proceed to purchasing.
“We need to be very mindful that a pre-purchase agreement is dependent on the satisfactory completion of the large phase three studies which are still underway. These results need to be well scrutinised by New Zealand’s licensing authority Medsafe to ensure the safety and effectiveness profile of each vaccine. Other countries such as the USA and the UK have gone ahead with emergency approval on intermediate results which are looking good, and starting to deliver vaccines, but they are in a different position from NZ. While economically it is very challenging for us to have border controls, we currently do not have severe illness and death from this disease, a very privileged position. I personally feel that it is appropriate for us in our current situation to be securing supplies of vaccine, but awaiting the final results of the studies.
“I am very pleased to see NZ is also committing to supporting our Pacific neighbours. Many wealthier countries, like NZ, are buying up early supplies of vaccine which is reducing the world stock for the Covax initiative, making vaccine availability for lower income countries further off. At least NZ and Australia are committing some support for our less resourced neighbours and continuing to support Covax.
“Vaccines are not suddenly going to get to New Zealand’s shores. We now will have a period of at least three, but more likely nearly six months to continue to scrutinise the science, watch how the vaccine rollout goes for other countries and prepare at our end. By the time vaccines enter NZ we will have more safety data, and particularly extra data from the monitoring systems internationally as vaccines are rolled out elsewhere which will be very useful.
“The planned approach by NZ recognises we have two issues. Firstly is to protect individuals as fast as possible, hence the logic behind the Ministry of Health’s focus on protecting those managing borders and quarantine services first, followed by front line providers. The second issue is how to consider opening up our borders, and this still looks a way off. As yet we do not know if these vaccines have the ability to prevent transmission, and that is a crucial piece of information we will need as we plan ahead.
“So while this is not a magic bullet to restore life as we used to know it, it is a good starting plan, that makes rational scientist sense with the best knowledge we have today and a decent amount of sensible NZ caution.”
No conflict of interest declared.
Professor Graham Le Gros, Immunologist, Director Malaghan Institute of Medical Research; and Programme Director Vaccine Alliance Aotearoa New Zealand – Ohu Kaupare Huaketo, comments:
“This is excellent news for New Zealand, it means NZ will have a good range of vaccine types available for the population that will fit with our infrastructure and storage facilities. The variety of vaccine types that have been secured will ensure that we are not caught out if one of the vaccines fails to meet all the necessary requirements around efficacy and delivery.
“The careful process being followed before roll out will mean that nothing is being rushed and the vaccines will only be administered once all the safety data has been reviewed by NZ’s regulatory agency Medsafe, and the personnel and resources are in place for the actual vaccine. This is good news for New Zealand and the Pacific.”
Conflict of interest statement: Professor Le Gros is Programme Director of the Government-funded Vaccine Alliance Aotearoa New Zealand – Ohu Kaupare Huaketo, a partnership between the Malaghan Institute, the University of Otago and Victoria University of Wellington.
Associate Professor Siouxsie Wiles, School of Biological Sciences, University of Auckland, comments:
“Today’s announcement of the purchase of enough COVID-19 vaccine doses to vaccinate every New Zealander, and some of our Pacific neighbours, is very good news. The Government have taken the wise approach to invest in a portfolio of vaccines that have been developed using a range of technologies. This is important because each technology has different advantages and disadvantages, and some vaccines will be more acceptable to some communities than others. Some may yet also fail to be found to be effective enough to roll out in New Zealand.
“The fact that Medsafe are accepting rolling submissions of safety and efficacy data also means that New Zealanders can be assured that there will be no unnecessary delays in authorising the vaccines should that data prove they are safe and effective.
“The big question now is, how safe and effective are the different vaccines? While data on safety looks really good, the scientific community are still waiting to see all of the data for how well each vaccine prevents transmission of the COVID-19 virus as well as preventing severe disease. That will determine how the vaccines should be rolled out in New Zealand and when it will be safe to open our borders again.”
No conflict of interest.
Note: Siouxsie Wiles and Toby Morris have created a GIF on the different vaccine technologies being used for a COVID-19 vaccine. This is available under a Creative Commons licence so can be edited/reused but must retain their names and that of the Spinoff.
Professor David Murdoch, Dean and Head of Campus, University of Otago, Christchurch, comments:
“The New Zealand COVID-19 vaccine portfolio now includes three different vaccine technologies. This aligns with other diverse country portfolios from Australia and several from North America and Europe, and provides for alternatives should some options not progress after clinical trials are completed.
“Today’s announcement also provided details on the sequencing plan for the vaccine roll out, planned to commenced in the second quarter of 2021. Border and managed isolation and quarantine workers, health workers at highest risk of exposure to COVID-19, and household contacts of the first two groups will be the first group to receive the vaccine.
“This will be by far New Zealand’s largest ever immunisation roll out, requiring the alignment of multiple systems to ensure successful and safe coverage. Today’s announcement indicated that the programme is progressing well, but this will required continued focused effort for some time to come.”
Conflict of interest statement: Member, COVID-19 Vaccine Strategy Taskforce, NZ Government. Member, COVID-19 Vaccine Strategy Scientific and Technical Advisory Group, NZ Government. Member, Advisory Group, Vaccine Alliance Aotearoa New Zealand (VAANZ). Independent Member, Clinical Trials Steering Committee, University of Oxford COVID-19 Vaccine. Member, COVID-19 Expert Advisory Network, Ministry of Health.
Associate Professor James Ussher, Immunologist, Clinical Microbiologist, Director Webster Centre for Infectious Diseases University of Otago; and Science Director Vaccine Alliance Aotearoa New Zealand – Ohu Kaupare Huaketo, comments:
“It is very encouraging to see the progress the Government has made in accessing vaccines for New Zealand. Along with participation in the COVAX Facility, the vaccine portfolio that has been assembled provides increased certainty that New Zealanders will have access to effective vaccines, starting from Q2 2021.
“The portfolio includes several technologies, including mRNA, viral vectors, and a protein subunit vaccine. All candidates are in advanced phase clinical development. Having a diverse portfolio insures us against failure of particular candidate during clinical trials and maximises the chances that a suitable vaccine will be available for most New Zealanders. Along with the Janssen vaccine, the AstraZeneca and Novavax vaccines are more straightforward to store and deploy than Pfizer’s mRNA vaccine; as such these vaccines should be suitable for deployment in the Pacific.
“It is also very encouraging to see the Government’s ongoing commitment to the Pacific and to global vaccine access. All countries should have access to effective vaccines at the earliest opportunity and New Zealand’s contribution to COVAX will assist with this.”
Conflict of interest statement: Associate Professor Ussher is Science Director of the Government-funded Vaccine Alliance Aotearoa New Zealand – Ohu Kaupare Huaketo, a partnership between the University of Otago, the Malaghan Institute and Victoria University of Wellington. He is also on the Government Vaccine Taskforce’s Science and Technical Advisory Committee.
Associate Professor Helen Petousis-Harris, vaccinologist, University of Auckland, comments:
“This is a great note to end the year on. NZ now has agreements with a diverse range of vaccine manufacturers that includes enough vaccine for all who want it. There are still plenty of unknowns.
“The RNA vaccines, like the Pfizer vaccine, have set a very high bar indeed in terms of their efficacy at well over 90%. It is unreasonable to expect that all vaccines will be able to achieve this level of protection. Like with any new vaccine we must wait until the product has been in use for a while before we know how long it protects for and how well it prevents transmission of the virus and therefore the impact on community immunity. Be patient, this will come.
“The AstraZeneca vaccine has shown it is protective in Phase III trials. However, there is less certainty about the estimates compared with the Pfizer and Moderna results for a couple of reasons. One, there are fewer numbers of participants included in the results so far which means less precision. Two, their results are based on several trials that have some subtle differences. What this means is that some more data is required in order to be more precise about the protective efficacy. We also look forward to more information about the optimal dosing regime.
“There are no Phase III results yet for the Janssen or Novavax candidates. My feeling is that now we see effectiveness for the mRNA and viral vector candidates there is a reasonable chance that these vaccines will also be successful.
“In terms of safety: Given the many COVID vaccines in play right now and the hundreds of thousands of doses administered, there have been very few ‘haltings’ to investigate safety concerns. Overall this also bodes very well so far for the safety of COVID vaccines. As they are deployed safety will be monitored very, very closely.
No conflict of interest declared.